Enhancing interdisciplinary research is a strategic goal for the University. An important component of this is the strengthening the inter-campus collaborations between Storrs and UConn Health. We can assist faculty in preparing and submitting externally funded inter-campus proposals and awards.
Because UConn (Storrs) and the UConn Health (UCH) are two separate fiscal entities, proposals seeking extramural funds are administered similar to sponsored activity involving any other inter-institutional collaborations – where one institution serves as the lead applicant and the other consortium partner acts as the subawardee.
This section of the website provides educational information and support including tutorials, manuals, job aides, videos, and other reference guides to help and support faculty, staff, and students.
Frequently Asked Questions
Below are some frequently asked questions regarding cross campus collaborations. Each section also contains links for webpages and contact information.
Financial Conflict of Interest
Question: How is the financial conflict of interest disclosure requirement on a proposal handled when there is a cross-campus collaboration involving a faculty member with a dual appointment at UConn Health and UConn (Storrs, Regionals, School of Law, School of Social Work)?
Answer: A faculty member may be considered compliant with the financial disclosure requirement as long as the faculty member has a current financial disclosure on record at the campus of their primary department/school. FCOI training is incorporated into the disclosure forms at both campuses.
Question: How is the financial conflict of interest disclosure requirement on a proposal handled when there is a cross-campus collaboration involving a faculty with dual appointment at UConn Health or UConn and another local institution (e.g., JAX, CCMC)?
Answer: The University FCOI policy applies to faculty members involved in research activities at the University. Thus, they must have a current financial disclosure on file within the University’s electronic disclosure submission system at the campus of their department/school.
Question: How are reviews of potential financial conflicts of interests for cross-campus research handled?
Answer: All University faculty investigators responsible for research activities on a cross-campus research project would have a current financial disclosure on record. The Financial Conflict of Interest in Research Committee (FCOIRC) at the campus of the faculty members’ department/school would review any disclosed significant financial interests when the project involves research activity at that campus.
Institutional Animal Care and Use Committee (IACUC)
Question: If there’s a collaboration using animals, who needs to hold the protocol?
Answer: There should be an animal protocol in place at the campus where the work will be performed. Personnel from the collaborating campus can be listed on that protocol.
Question: If the collaboration requires an animal to be used at both institutions as part of the study, who needs to hold the protocol and who owns the animal?
Answer: A protocol should be in place at each institution to describe the work that is specific to that site. The overarching collaboration should also be described in both protocols, and animal ownership should be transferred when an animal is moved to the secondary institution.
Question: If I will be listed on a protocol at my collaborator’s institution, will my home campus training be valid at the collaborating institution?
Answer: It depends. The general IACUC training and Occupational Health and Safety Program enrollment is portable, but training specific to the institution where the animal work will be performed may also be necessary (e.g. facility orientation).
Question: If there is a PHS funded grant describing a collaboration between the two campuses, who is responsible for assuring congruency between the grant and the animal protocol?
Answer: The “Prime” institution has the responsibility of assuring congruency.
Institutional Biosafety Committee (IBC)
Question: I am a PI at UCH, but have some research that is conducted at UConn (Storrs and/or regionals). Does my UCH IBC registration cover my research at UConn?
Answer: No. UConn and UCH have separate IBCs, with different requirements. Therefore IBC registration processes are different between the two campuses. If research is being conducted at UConn by a UCH PI, please contact ibc@uconn.edu, for additional information and assistance in completing the appropriate registration forms. If you are a UConn PI, contact rwallace@uchc.edu .
Question: I have samples coming from UCH, and my personnel are conducting the analysis at UConn. Which IBC do I need to contact to complete a registration?
Answer: If the samples are being analyzed at UConn, an IBC registration will need to be completed through the UConn IBC. If there are any analyses being conducted at UCH, the UCH IBC should be contacted to determine if an IBC registration is necessary.
Question: I am a PI at UCH, but I teach a course at UConn which includes lab teaching activities that involve biological materials. Do I need an IBC registration with the UConn IBC or UCH IBC?
Answer: A UConn IBC registration is required for these activities. At UConn, all experimental or teaching activities involving biological materials must be registered with the UConn IBC. It should be noted that biological materials includes but is not limited to: recombinant or synthetic nucleic acid molecules (rsNA), bacteria and their phages and plasmids, viruses, biological toxins, fungi, mycoplasmas, prions, and parasites; human and non-human primate tissues, body fluids, blood, blood byproducts, and cell lines, transgenic and wild type animals and plants, animal remains and insects that may harbor zoonotic pathogens. Teaching activities being conducted at UCH may require an IBC registration. The UCH IBC should be contacted to determine if an IBC registration with them is necessary.
Question: I have already taken Biosafety and Bloodborne Pathogens Training at UCH. Do I have to take these trainings at UConn as well?
The training section references Blood Borne Pathogen training specifically, however, there are additional training such as Initial Lab Safety training that are required to conduct research at both locations, so perhaps the language can be shifted to something more neutral such as Each institution offers distinct training packages, please reach out to EH&S at each facility to make sure training requirements are met. Or something similar.
Question: I am a researcher using human embryonic stem cells (hESC) and/or human induced pluripotent stem cells (hiPSC). Do I need to register both with the Stem Cell Research Oversight committee (SCRO) and the IBC?
Answer: It depends. For work at UConn, you need to register with both the SCRO and IBC. At UCH for hESC, you need to register with the SCRO but not the IBC if your experiment is exempt under the NIH Guidelines. If you will be using animals or viral vectors with the cells it is definitely not exempt and you will need to register with the respective (UConn or UCH) IBC. For other experiments with rsNA, it’s always a good idea to consult with the respective IBC. As with any human cells, you need to work at BSL-2 containment and have fulfilled Bloodborne Pathogen (BBP) training and HBV immunization requirements (documented titer or declination). For hiPSC it’s the same as hESC except for certain uses no SCRO is required. Also, if the cells were reprogrammed with a HIV-based lentiviral vector that was not removed, the cell line will have remaining HIV sequences from the vector which are Risk Group 3 (RG-3) sequences. There is no exemption for working with (even just culturing) cells containing RG-3 rsNA sequences. hiPSC reprogrammed with Sendai vectors or plasmid vectors eventually contain no rsNA and are technically not recombinant, though they remain human and fall under the BBP rules. hiPSC reprogrammed with retroviral vectors have residual viral sequences (not RG-3). They are recombinant and fall under the exemption for culturing, but certain other experiments, such as transfer into animals require an IBC registration.
Human Subjects Research
Human Subjects Research Website
Human Subjects Research Contacts
Guidelines for Collaborative Research
When UConn Storrs and UConn Health faculty or personnel are collaborating on a research study, the IRB offices will determine the appropriate IRB of record. The lead Principal Investigator on the project should contact their institution’s IRB office as early in the planning process as possible and before submitting the study to either IRB for review. Storrs PIs should email irb-reliance@uconn.edu. PIs at UConn Health should email the UConn Health IRB at irb@uchc.edu. In the body of the email, PIs should provide their IRB with a brief description of the study, a list of UConn Health and Storrs personnel who will be engaged at each site, and a description of the research activities that will occur at each site.
Cross campus recruitment: Requests for permission for external researchers to recruit UConn faculty, students, staff, or UConn Health patients, faculty, students or staff for research must be submitted via a web-based request form located on the Storrs IRB Contacts webpage and the UConn Health IRB Instructions, Forms, and Samples webpage. This process also applies to cross-campus requests to recruit (i.e., permission for Storrs and Regional Campuses researchers to recruit at UConn Health and vice versa). The request form will be routed to appropriate offices and departments for review.
Use of UConn Health Facilities: Researchers who want to use UConn Health facilities to conduct Storrs IRB-approved research activities in UConn Health facilities will need to request permission by submitting a Confirmation of Available Resources form to irb@uchc.edu.
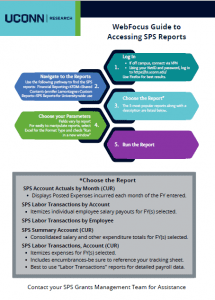
Sponsored Program Services (SPS)
SPS Contacts:
This page is under construction.