Revision Date: March 8, 2021 – revisions in italics
As an applicant organization, UConn/UConn Health has an institutional responsibility to verify the accuracy, validity, conformity, and eligibility of all applications submitted to a sponsor on behalf of the University. We have been exploring how to ensure the best delivery of services, meet sponsor and institutional policy requirements that were highlighted in the recent NSF audit, and to ensure that the University has sufficient time to review and certify proposals and increase the number of successful applications. To that end, we have conducted a survey of all faculty who submitted grant proposals within the last two years, listened to the research community’s comments at town halls, and solicited input from the President, Provost, deans, associate deans for research, the President’s Research Advisory Council, University Senate, and other faculty groups.
This listening process revealed that one of the main challenges for both investigators and staff is the bottleneck that occurs immediately prior to proposal submission. In recent years, nearly two-thirds of proposals submitted (with all components ready) are received by Sponsored Program Services (SPS) within one working day or less of the sponsor deadline.
Numerous proposals are being submitted just barely in time, meaning there is little time for a thorough review. Additionally, proposals that have been submitted to SPS far in advance also routinely lack a timely and thorough review because other proposals with an earlier deadline came in and “cut the line.”
To begin to remedy the proposal submission bottleneck, beginning May 5, 2021, the Office of the Vice President for Research (OVPR) will implement the current policy on internal deadlines for the review and submission of sponsored project proposals. The process change aims to reduce last day proposal submissions and will prioritize proposals as received. Once the policy is implemented, final administrative components of a proposal must be received by SPS Pre-Award at least five full business days in advance of the submission due date (along with a draft of the scientific components). The final submission ready proposal is due to SPS no later than noon the day before the sponsor deadline.
Exceptions include short turnaround RFPs, last minute sponsor requests, or a last minute opportunity to join a proposal under submission by another institution. Also, each UConn investigator will be given one pass to use in the event they are not able to meet the internal five-day deadline. As is the current practice, SPS Pre-Award will make every effort to submit these proposals when possible. Please visit the OVPR website for additional information and FAQs regarding the internal deadline policy.
To increase faculty support related to proposal preparation, the OVPR will be taking the following additional steps:
- The OVPR is working to address situations where investigators do not have dedicated administrative support for the preparation of a proposal; we will continue to increase staff training opportunities, extend faculty service offerings, and work to simplify the submission process.
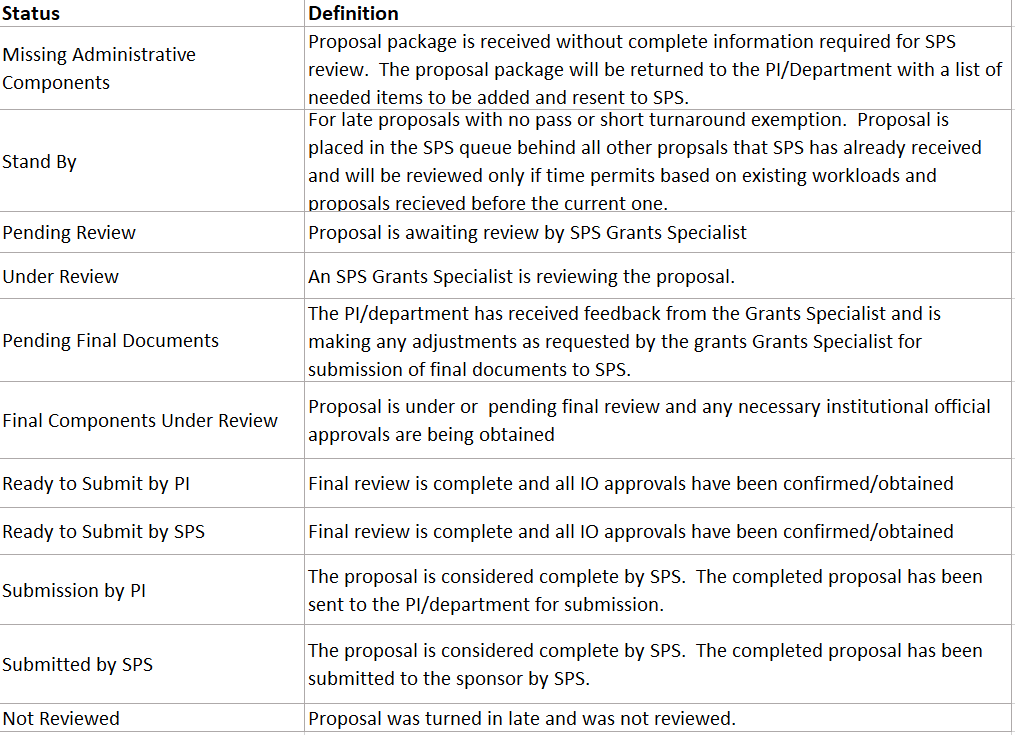
- The OVPR will implement a dashboard to increase transparency and provide information on the status and order of review.
- The OVPR will provide additional research development services, such as grant editing and proofreading, proposal review, large and complex grant support, and research funding consultation. Further information regarding these services and how to request them is available on the OVPR Research Development section of this website.
- The OVPR will continue to work with and incorporate feedback from faculty working groups. Upon recommendation of the University Senate, the President formed a sponsored projects working group to identify impediments to the expeditious review of sponsored project proposals in advance of deadlines. The group’s report is available on the University Senate website.
Development, review, and submission timeline:

Full Business Days Before Submission Deadline
>6 days: PI provides application components to local grants administrator (or Faculty Services)
5 days: Complete application (plus draft scientific components) and IPR submitted to SPS
5-2 days: SPS reviews proposal and provides feedback
2 days: Corrections made and all approvals in place
Noon day before deadline: Final proposal and PI authorization to submit to sponsor provided to SPS
1-0 days before deadline: Proposal submitted
SPS is responsible for ensuring that applications are compliant and that institutional and sponsor guidelines are met including administrative, management, and scientific information. Please contact Paul Hudobenko (hudobenko@uchc.edu/UConn Health) or Mark Reeves (mark.reeves@uconn.edu/Storrs and Regionals) with questions as we move to a consistent and sustainable process.
Thank you for your continued cooperation in our collaborative efforts to advance UConn’s mission through innovative research, scholarship, and creative pursuits.