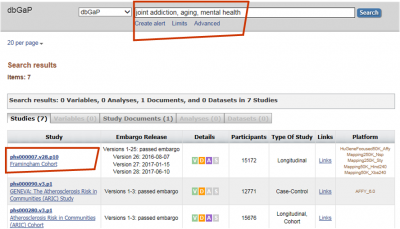
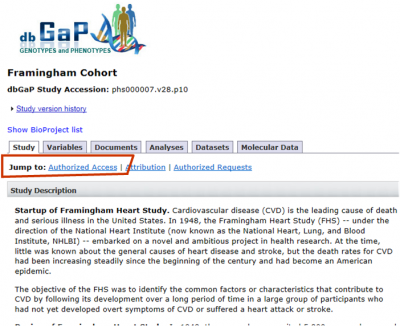
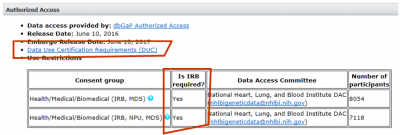
INTERNATIONAL RELATIONSHIPS, FOREIGN COMPONENTS, AND SPONSORED PROGRAMS
It protects everyone’s interests – those of the Federal government, UConn, individual investigators, and their international collaborators – to have international relationships disclosed and vetted to determine if there are any potential conflict of commitments, duplications of research, and/or diversion of intellectual property in the performance of federally funded research. In some cases, failure to disclose all relationships could result in the termination of funding for a project and potential ineligibility for future funding. Noncompliance can also threaten not only the funding for individual projects, but overall funding for the University. UConn encourages international collaborations and the OVPR strongly urges investigators to err on the side of transparency in disclosing these collaborations to the University and Sponsors.
MALIGN FOREIGN TALENT RECRUITMENT PROGRAMS
Beginning 8/9/2024, all federal agencies must prohibit participation in a malign foreign talent recruitment program by covered individuals involved with research and development awards from those agencies.
UPDATE 8/7/2024: UConn has established a Malign Foreign Talent Program Participation Policy, which requires that Covered Individuals listed in a proposal for a research award from a Federal Sponsor must certify as part of the proposal submission that they are not currently participating in, nor will they participate in, a Malign Foreign Talent Recruitment Program.
Foreign Talent Recruitment Programs (Section 10631(b), Creating Helpful Incentives to Produce Semiconductors [CHIPS] and Science Act of 2022)
Section 10631(d) of the CHIPS and Science Act of 2022 requires federal research agencies to issue a policy on foreign talent recruitment programs, along with a policy specific to malign foreign talent recruitment programs. The Act prohibits “covered individuals” from participating in a federally funded research and development project if they are currently participating in a “malign foreign talent recruitment program.” Federal research agencies may request supporting documentation from applicants, and take a range of funding-related actions. The Act defines “covered individual” as “an individual who A. contributes in a substantive, meaningful way to the scientific development or execution of a research and development project proposed to be carried out with a research and development award from a Federal research agency; and B. is designated as a covered individual by the Federal research agency concerned.” (Agencies may define other individuals as covered persons as appropriate and consistent with their mission.
A malign foreign talent recruitment program is any type of program, position, or activity that involves one or more of the following:
- Unauthorized transfer of intellectual property, materials, data or other nonpublic information;
- Recruitment of trainees or researchers to enroll in such program, position or activity;
- Establishing a laboratory or entity in a foreign country in violation of terms and conditions of a federal research award;
- Accepting a faculty position, or undertaking any other employment or appointment in violation of the standard terms and conditions of a federal research award;
- Being unable to terminate the activity except in extraordinary circumstances;
- Being limited in capacity to carry out a federal research award;
- Requirement to engage in work that overlaps or duplicates a federal research award;
- Requirement to obtain research funding from the foreign government’s entities;
- Requirement to omit acknowledgement of the U.S. home institution and/or the federal funding agency;
- Requirement to not disclose participation in the program, position, or activity; OR
- Having a conflict of interest or commitment contrary to a federal research award.
AND is sponsored by one of the following:
- A foreign country of concern (currently defined as the People’s Republic of China including Hong Kong and Macau, the Democratic People’s Republic of Korea, the Russian Federation, the Islamic Republic of Iran, or any other country determined to be a country of concern by the Secretary of State); or
- An entity based in a foreign country of concern; or
- An institution or program on certain prohibited lists (contact the Director of Research Integrity and Security for screening).
AGENCY-SPECIFIC GUIDANCE
National Science Foundation
Individuals who are a current party to a Malign Foreign Talent Recruitment Program are not eligible to serve as a senior/key person on an NSF proposal or on any NSF award made after May 20, 2024. As of the effective date of May 20, 2024 of NSF 24-1 Proposal and Award Policies and Procedures Guide (PAPPG), the Authorized Organizational Representative (typically the Pre-Award Senior Grants and Contracts Specialist) must certify that all individuals identified as senior/key personnel have been made aware of and have complie3d with their responsibility to certify that the individual is not a party to a malign foreign talent recruitment program. IN ADDITION, each identified senior/key person must certify, in signing their Biographical Sketch form via SciENcv (required), that they are not a party to a malign foreign talent recruitment program and annually thereafter for the duration of the award.
Misrepresentations and/or omissions may be subject to prosecution and liability pursuant to, but not limited to, 18 U.S.C. §§ 287, 1001, 1031 and 31 U.S.C. §§ 3729-3733 and 3802.
National Institutes of Health
Full transparency in NIH applications and throughout the life of an NIH grant is critical. NIH requires the disclosure of all sources of research support, foreign components, and financial conflicts of interest for senior/key personnel on research applications and awards. NIH uses this information when making its funding decisions to determine if the research being proposed is receiving other sources of funding that could be duplicative, has the necessary time allocation, or if financial interests may affect objectivity in the conduct of the research.
NIH Requirements for Disclosure of Other Support, Foreign Components and Conflicts of Interest
NIH Decision Matrix for Assessing Potential Foreign Interference for Covered Individuals or Senior/Key Personnel
Reminders of NIH Policies on Other Support and on Policies related to Financial Conflicts of Interest and Foreign Components (NOT-OD-19-114)
Department of Defense
Beginning August 9, 2024, the Department of Defense is prohibited from providing funding to or making an award of a fundamental research project proposal in which a covered individual is participating in a malign foreign talent recruitment program or to a proposing institution that does not have a policy addressing malign foreign talent programs pursuant to Section 10632 of the CHIPS and Science Act of 2022.
On March 20, 2019, DoD issued a memo directing that all new DoD Notices of Funding Opportunities pertaining to research and research-related educational activities include a requirement that proposers provide additional information on the other support and commitments of all key personnel, regardless of whether the proposal is funded.
Countering Unwanted Foreign Influence in Department-Funded Research at Institutions of Higher Education
DoD Academic Research Security
DoD Component Decision Matrix to Inform Fundamental Research Proposal Mitigation Decisions
Department of Energy
DOE Order No. O 486-1: Department of Energy Foreign Government Sponsored or Affiliated Activities prohibits DOE employees and contractors participating in foreign talent recruitment programs from certain countries from receiving DOE funding. Universities must therefore disclose whether a researcher currently involved in a DOE award has participated in either a funded or unfunded Foreign Government Talent Recruitment Program.
Additional Resources
White House Office of Science and Technology Policy Foreign Talent Recruitment Program Guidelines
Recommended Practices for Strengthening the Security and Integrity of America’s Science and Technology Research Enterprise (National Science & Technology Council)
GOVERNMENT CONCERN
- The National Institutes of Health (NIH) issued a Notice on March 30, 2018, reminding research institutions that PIs, sub-awardees and co-PIs must disclose all financial interests received from higher education or governmental institutions in countries outside the United States (NOT-OD-18-160). NIH Director Dr. Francis S. Collins also sent a memo.pdf to institutions on Aug. 20, 2018, stating that the failure to properly disclose foreign relationships threatened to distort decision-making about the use of NIH funds.
- The Department of Defense
- The National Defense Authorization Act, signed in August 2018, included Sec. 1286, which stated that “The Secretary of Defense shall, in consultation with other appropriate government organizations, establish an initiative to work with academic institutions who perform defense research and engineering activities . . . to limit undue influence, including through foreign talent programs, by countries to exploit United State Technology … ”
- Memorandum from the Office of the Under Secretary of Defense dated October 10, 2019 which states that “the challenge of protecting the integrity of our research enterprise is a national priority.” The letter lays out DoD’s steps to date to “limit undue influence by countries that desire to exploit DoD research, science and technology, and innovation enterprise through foreign talent programs and other means” and steps DoD plans to pursue, and calls for a dynamic, Government wide, partnership, “ No laboratory, university, industry partner, or Government agency can address the full scope of this challenge alone, and solutions to this problem can only result from a dynamic partnership between our public and private sectors.”
- Memorandum titled “Actions for the Protection of Intellectual Property, Controlled Information, Key Personnel and Critical Technologies” dated March 20, 2019. Directing for all new DoD Notices of Funding Opportunities related to research and research-related educational activities include requirements that “proposer submit additional Current and Active support information for all key personnel, whether or not the individuals are to funded by the DoD.
- The National Science Board issued a statement on “Security and Science” dated October 23, 2018, stating that US universities must “embrace transparency and rigorously adhere to conflict of interest and conflict of commitment policies.”
- The National Science Foundation, issued a letter on July 11 from the NSF Director to colleagues on “Research Protection” related to other support, financial conflicts of interest and foreign components (NSF 19-200).
- The Department of Energy issued a notification on February 1, 2019, stating that DOE plans to implement a policy, which will mandate that “federal and contractor personnel fully disclose and, as necessary, terminate affiliations with foreign government-supported talent recruitment programs.”
- As a reminder, NASA has long-standing restrictions on using NASA funds to enter into agreements “to participate, collaborate, or coordinate bilaterally in any way with China or any Chinese-owned company, at the prime recipient level or at any subrecipient level, whether the bilateral involvement is funded or performed under a no-exchange of funds arrangement” (grant restrictions, contract restrictions.pdf).
Foreign Components
Foreign components of federally funded research should be disclosed in proposals, progress reports, and final technical reports. Under the NIH Grants Policy Statement, a Foreign Component is defined as “any significant scientific element or segment of a project outside of the United States, either by the recipient or by a researcher employed by a foreign organization, whether or not grant funds are expended”. The definition of “foreign component” (which can be found here) may include a large number of collaborative activities, including “collaborations with investigators at a foreign site anticipated to result in co-authorship; use of facilities or instrumentation at a foreign site; or receipt of financial support or resources from a foreign entity.” Other sponsors have similar requirements to disclose foreign components.
NIH Policy on Foreign Component
NIH requires recipients to determine whether activities it supports include a foreign component, defined as the existence of any “significant scientific element or segment of a project” outside of the United States, in other words:
1. performance of work by a researcher or recipient in a foreign location, whether or not NIH grant funds are expended and/or
2. performance of work by a researcher in a foreign location employed or paid for by a foreign organization, whether or not NIH grant funds are expended.
- If a recipient determines that a portion of the project will be conducted outside of the U.S., the recipient then will need to determine if the activities are considered significant. If both criteria are met, then there is a foreign component. See NIH FAQs on Other Support and Foreign Components. The addition of a foreign component to an ongoing NIH grant continues to require NIH prior approval, as outlined in the NIHGPS (Section 8.1.2) Prior Approval Requirements.
- If an activity does not meet the definition of foreign component because all research is being conducted within the United States, but there is a non-U.S. resource that supports the research of an investigator and/or researcher, it must be reported as other support.
For example, if a PD/PI of an NIH-funded grant has a collaborator outside of the U.S. who performs experiments in support of the PD/PI’s NIH-funded project, this would constitute a foreign component, regardless of whether the foreign collaborator receives funding from the PD/PI’s grant. Additional funding from a foreign source for the NIH-supported research of a PD/PI at a U.S. institution would not constitute a foreign component but would necessitate reporting as other support.
- Foreign Other support must be disclosed to the NIH in advance by including it in the Just-in-Time Other Support submission on a new or renewal award, or updated in the annual RPPR, or submitted by letter to the awarding office and thereafter included in the RPPR.
- Foreign Components applies to NIH work scope performed in a foreign location, either funded by the NIH grant or by other sources, domestic or foreign.
Also see NIH’s “Protecting U.S. Biomedical Intellectual Innovation.”
Foreign Payments
The University’s Consulting Policy requires prior approval of any remuneration, compensation, honorarium, stipends, non-university salary, or other similar payments for providing consulting, advice, services, support or other similar activities from any source, including foreign entities or persons. The University’s Financial Conflicts of Interest in Research Policy requires disclosure of remuneration, compensation, honorarium, stipends, non-university salary, or other similar payments totaling over $5,000 in a 12 month period from a single entity, and any paid or reimbursed travel totaling over $5000 in a 12 month period by a single entity. This include any foreign entity (e.g. governments and institutions) or persons.
Current and Other Support
“Other Support” includes all financial resources, domestic or foreign, available in direct support of a researcher’s research endeavors. Such support should be disclosed on an “Other Support” or “Current & Pending” form. See here for guidance.
Past Communication
December 2020 Email from President Katsouleas: “Reminder: Threats to Research”
December 2019 Updates
How to Get Assistance?
Faculty and investigators who have questions or concerns about disclosure requirements should contact the OVPR for guidance and assistance.
Contact the OVPR

 The InfoEd eRA Portal is integrated with ORCID®. ORCID iDs are unique identifiers assigned to individual scholars and researchers. ORCID provides a persistent identifier – an ORCID iD – that distinguishes you from other researchers and a mechanism for linking your research outputs and activities to your iD. Using an ORCID allows your manuscripts, grants, and other scholarship to be more discoverable and integrated within larger research networks. Faculty, staff and students at the University of Connecticut
The InfoEd eRA Portal is integrated with ORCID®. ORCID iDs are unique identifiers assigned to individual scholars and researchers. ORCID provides a persistent identifier – an ORCID iD – that distinguishes you from other researchers and a mechanism for linking your research outputs and activities to your iD. Using an ORCID allows your manuscripts, grants, and other scholarship to be more discoverable and integrated within larger research networks. Faculty, staff and students at the University of Connecticut